In writing the electron configuration for Potassium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Potassium go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next. A step-by-step explanation of how to draw the KBr Lewis Dot Structure.For KBr we have an ionic compound and we need to take that into account when we draw th.
Potassium Valence Electrons How Many

Click to see full answer.
Regarding this, what is the principle quantum number for the valence electrons in K?
Correct answer:Potassium has one valence electron. This means that there is one electron in its outermost shell (4th shell). Potassium ion, on the other hand, loses an electron and has a complete octet (has eight valence electrons) in its 3rd shell. Recall that the principal quantum number signifies the shell.
Also Know, what are the quantum numbers for silicon? Quantum Numbers of the elements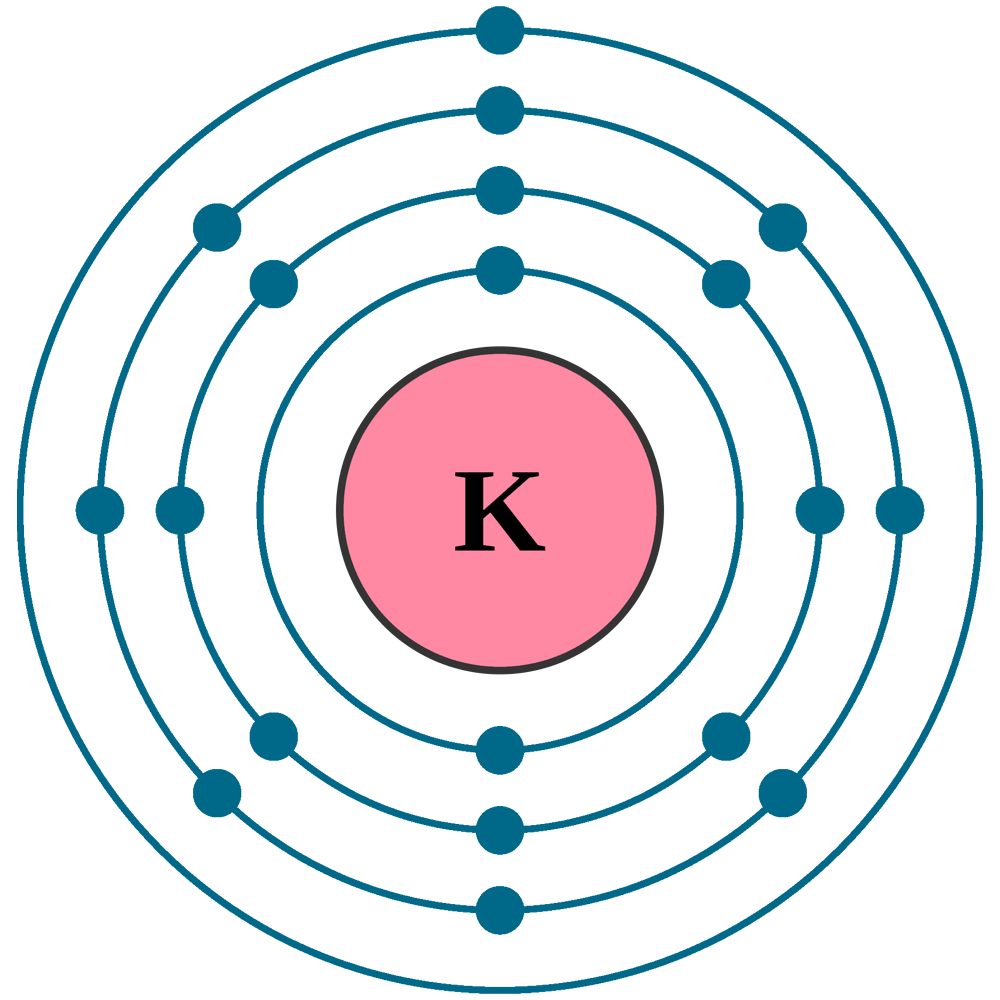
Potassium Ion Valence Electrons
| Hydrogen | 2S1/2 | 6D1/2 |
|---|---|---|
| Silicon | 3P0 | 1S0 |
| Phosphorus | 4S3/2 | 2S1/2 |
| Sulfur | 3P2 | 1S0 |
| Chlorine | 2P3/2 | 2D3/2 |
Keeping this in consideration, what is the ground state of potassium?
Potassium atoms have 19 electrons and the shell structure is 2.8. 8.1. The ground state electron configuration of ground state gaseous neutral potassium is [Ar]. 4s1 and the term symbol is 2S1/2.
What is the principal quantum number of potassium?
Explanation: The four quantum numbers for an atom refer to the state of the valence or outermost electron. For a potassium atom, the electron configuration is 1s22s22p63s23p64s1 , which means that n=4 .
